FOR HEALTHCARE PROVIDERS
Study enrollment
In line with FDA recommendations, “Patients may be eligible for inclusion in trials of investigational drugs, including first-in-human trials, regardless of whether they have received available therapy in the non-curative setting.”1
We are actively recruiting patients for our pre-screening, observational study BASECAMP-1, to identify participants who may benefit from future Tmod™ CAR T-cell therapy in our planned investigational studies.
BASECAMP-1: Study rationale
Importance of HLA LOH identification
Due to an irreversible, clonal loss within tumor cells, human leukocyte antigen (HLA) loss of heterozygosity (LOH) can be used to distinguish between tumor and normal tissue.2,3
The TmodTM CAR T-cell platform is composed of two receptors:
- An activator that recognizes an antigen present on the surface of normal and tumor cells
- A blocker that recognizes a second surface antigen for an HLA allele only lost in tumor cells
Frequency of HLA-A LOH in advanced tumorsa
In the Tempus real-world database, LOH occurs in between 12.2% and 26.0% of advanced solid tumors with an average of 16.3% in 10,867 samples tested.4
| Tempus HLA-A LOH advanced disease in the real world | The Cancer Genome Atlas Program (TCGA) HLA-A LOH primary tumors5 | |
|---|---|---|
| Average | 16.3% (n=10,867) | 12.6% (n=10,844) |
| Non-small cell lung cancer | 23.1% (n=1,915) | 25.3% (n=501) |
| Head and neck cancer | 26.0% (n=208) | 16.1% (n=522) |
| Breast cancer | 12.2% (n=1,447) | 13.6% (n=1,080) |
| Ovarian cancer | 16.0% (n=569) | 17.1% (n=579) |
| Colorectal cancer | 15.6% (n=1,854) | 9.6% (n=615) |
| Pancreatic cancer | 19.6% (n=675) | 33.1% (n=184) |
| Gastroesophageal cancer | 20.8% (n=506) | 16.2% (n=625) |
| Mesothelioma | 14.3% (n=7) | 11.5% (n=87) |
aTempus data contain more advanced disease, and TCGA data have more primary tumors.
Figure adapted from Simeone DM, et al.6
Utilizing next-generation sequencing (NGS) to identify LOH
Tempus assay for LOH
HLA-A*02 LOH can only be therapeutically utilized if patients are identifiable through a feasible and timely clinical workflow.
The Tempus xT is a solid tumor + normal match DNA sequencing platform, which utilizes the patient’s own genome as a comparator, resulting in a personalized sequencing analysis.7 The assay uses archived, formalin-fixed, paraffin-embedded primary tumor tissue with matched-normal (germline) samples.
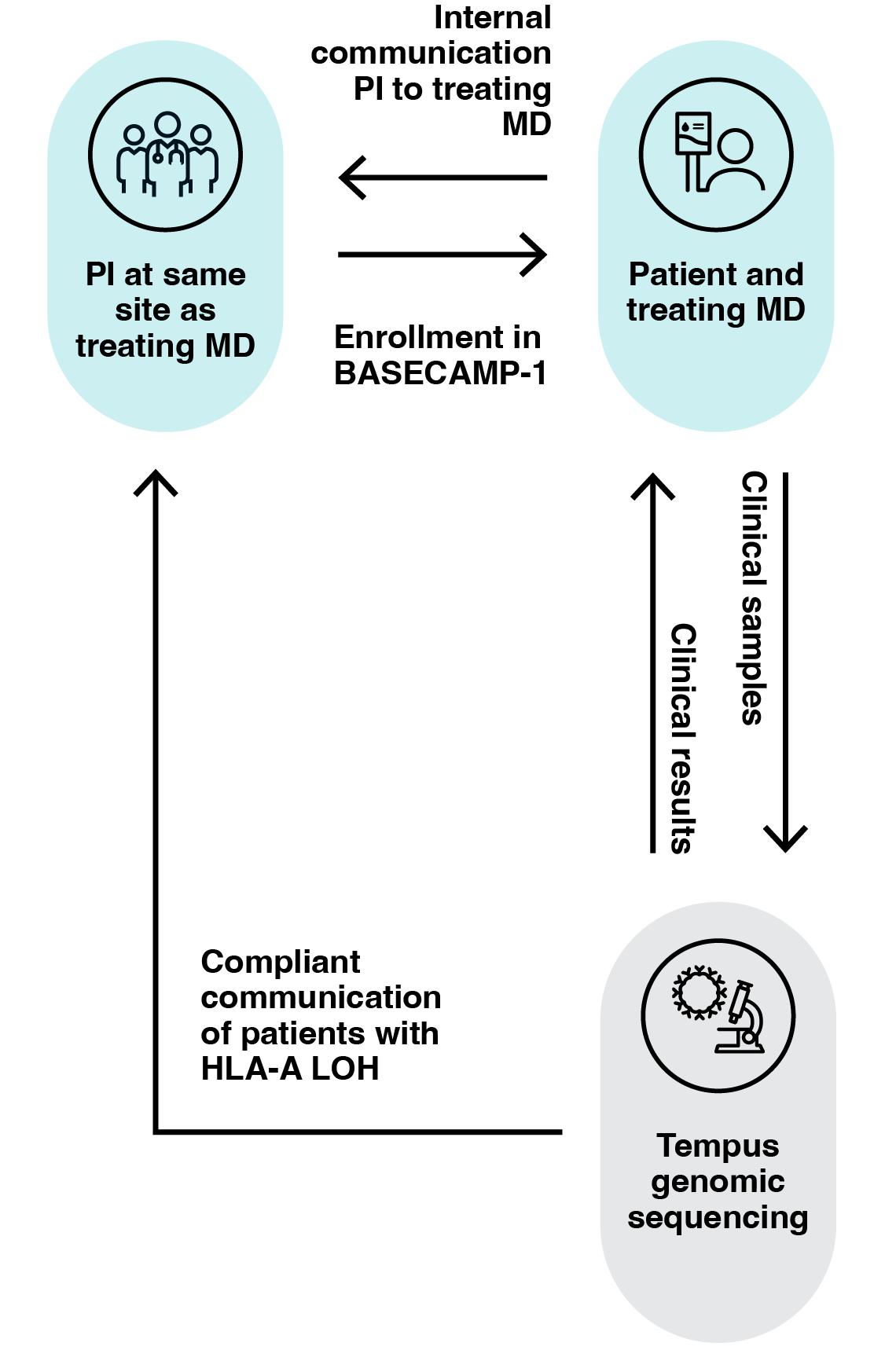
Tempus clinical workflow
Tempus xT can identify HLA LOH within a clinically feasible workflow and timeframe.8
Smart trial matching tools
A2 Bio uses Tempus tools to help increase awareness of BASECAMP-1 at and around our existing study centers.8
BASECAMP-1: Key study details
Study schema

Biospecimen retention
Samples with DNA
- Blood, saliva, or buccal swabs will be obtained to determine germline HLA type and for tumor comparison
- Archived tumor tissue samples will be obtained for NGS to determine LOH status of tumor tissue
- DNA and RNA will be retained only for enrolled participants if repeat testing is required. No further genetic testing will be performed on these samples
- Peripheral blood mononuclear cells (PBMCs) will be collected for enrolled participants, enriched for T cells, and cryopreserved for future manufacturing of an A2 Bio CAR T-cell therapy upon participant relapse. No further genetic testing will be performed on this sample
- Tumor slides will be obtained for immunohistochemistry
Further information about this study can be found here: NCT04981119
If you would like to discuss enrolling a patient into BASECAMP-1, or have further questions, please contact us: clinicaltrials@a2bio.com or (310) 431-9180.
Current studies
Explore additional information on our ongoing clinical studies that are recruiting patients.
Pre-screening study
BASECAMP-1
Aims to find patients who might be suitable for treatment with an investigational therapy in one of our clinical studies. For adults with mesothelioma or colorectal, non-small cell lung, pancreatic, or ovarian cancers; or other solid tumors that have lost HLA-A*02 expression.
Interventional studies
EVEREST-1
EVEREST-1 has now closed enrollment. Further information about this study can be found here:
EVEREST-2
Aims to understand the safety and efficacy of an investigational TmodTM CAR T-cell therapy (A2B694) in germline heterozygous HLA-A*02 adults with mesothelioma, colorectal, non-small cell lung, pancreatic, or ovarian cancers; or other solid tumors that express the biomarker MSLN and have lost HLA-A*02 expression.
DENALI-1
Aims to understand the safety and efficacy of an investigational Tmod CAR T-cell therapy (A2B395) in germline heterozygous HLA-A*02 adults with colorectal cancer, non-small cell lung cancer, head and neck squamous cell carcinoma, triple-negative breast cancer, renal cell carcinoma, or other solid tumors that express the biomarker EGFR and have lost HLA-A*02 expression.
Abbreviations
CAR, chimeric antigen receptor; CEA, carcinoembryonic antigen; HLA, human leukocyte antigen; MSLN, mesothelin.
References
-
- Food and Drug Administration (FDA). Cancer Clinical Trial Eligibility Criteria: Available Therapy in Non-Curative Settings Guidance for Industry. July 2022.
Available at: https://www.fda.gov/media/150244/download. Accessed October 2023. - Hamburger A, et al. Engineered T cells directed at tumors with defined allelic loss. Mol Immunol. 2020;298–310.
- Hwang MS, et al. Targeting loss of heterozygosity for cancer-specific immunotherapy. Proc Nat Acad Sci U S A. 2021;118(12):e2022410118.
- Hecht J, et al. Next generation sequencing (NGS) to identify relapsed gastrointestinal (GI) solid tumor patients with human leukocyte antigen (HLA) loss of heterozygosity (LOH) for future logic-gated CAR T therapy to reduce on target off tumor toxicity. J Clin Oncol. 2022;40(4_suppl):190–190.
- The Cancer Genome Atlas (TCGA) Research Network. Available at: https://www.cancer.gov/tcga. Accessed October 2023.
- Simeone DM, et al. BASECAMP-1: Leveraging HLA-A loss of heterozygosity in solid tumors by NGS to identify patients with relapsed solid tumors for future CEA and MSLN logic-gated Tmod™ AR T-cell therapy. Presented at the Society for Immunotherapy of Cancer (SITC) 37th Annual Meeting. Boston, MA, USA. November 08–12, 2022. Abstract #639.
- Tempus genomic profiling: xT and xR. Available at: https://www.tempus.com/oncology/genomic-profiling/xt-xr/. Accessed October 2023.
- Hecht RJ, et al. Prospective BASECAMP-1 experience in patients with gastrointestinal (GI) cancer: Identifying patients with human leukocyte antigen (HLA) loss of heterozygosity (LOH) for a future therapeutic trial exploiting LOH as a tumor vulnerability. Presented at the ASCO Gastrointestinal (GI) Cancers Symposium. Moscone West, San Francisco, CA, USA. January 19–21, 2023. Abstract #209.
- Food and Drug Administration (FDA). Cancer Clinical Trial Eligibility Criteria: Available Therapy in Non-Curative Settings Guidance for Industry. July 2022.